Keeping Compliant with OSHA’s Bloodborne Pathogens Standard when Using Scalpels
Sharps cuts and injuries from scalpels, suture needles, and other sharps are a significant occupational hazard for staff in hospitals and healthcare facilities, leading to long-term impacts on physical and psychological health. This is especially true for those working in Operating Rooms, which are high-pressure environments where sharps are used frequently. To combat occupational injuries, regulations have been mandated by OSHA under The Bloodborne Pathogens Standard (29 CFR § 1910.1030). This guide aims to help facilities using scalpels make sure that they are up-to-date with their OSHA compliance, and keeping staff safe.
What is the Bloodborne Pathogens Standard?
The Bloodborne Pathogens Standard (29 CFR § 1910.1030) is the OSHA regulation designed to protect workers in the USA against occupational exposures to bloodborne pathogens including Hepatitis B (HBV), Hepatitis C (HCV), and HIV. Compliance to the Bloodborne Pathogens Standard is mandatory for all workplaces where employees may be exposed to human blood, including healthcare facilities like hospitals, outpatient surgical centers, and dental clinics, as well as other workplaces such as laboratories and colleges.
The standard was first enacted by OSHA in 1992 and called for workplaces to implement risk-control measures to prevent exposures to bloodborne pathogens. It was revised following the passing of the Needlestick Safety and Prevention Act of 2000. The revised standard specified further requirements of workplaces to prevent sharps injuries, including the implementation of safety-engineered devices and a sharps injury log.
OSHA continues to publish Standard Interpretations in response to public inquiries into the Bloodborne Pathogen Standard. These Interpretations are useful for facilities to understand how emerging evidence-based practices and safety-engineered devices apply to the standard’s requirements.
What policies do I need in place to be compliant?
Under the standard, a written Exposure Control Plan must be developed and implemented by healthcare facilities and other workplaces with potential exposures to bloodborne pathogens. The facility must identify which employees (both clinical and non-clinical) are at risk of occupational exposure. In facilities where scalpels are used, the employees exposed to the hazard include those who handle scalpels (e.g. perioperative nurses, surgeons, surgical technicians, pathologists, etc) and downstream workers who clean up after procedures (e.g. laundry workers). It’s important to note that employees are considered to be exposed even if they are using personal protective equipment (PPE).
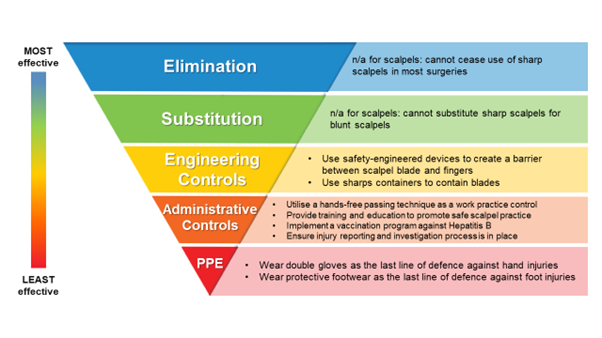
The Exposure Control Plan must outline what preventative control measures are implemented in the facility to meet the requirements of the standard, including who is responsible for the implementation and recording of specific measures. The Bloodborne Pathogens Standard follows the Hierarchy of Controls approach, where control measures are categorised and prioritised according to their effectiveness. An example of preventative control measures against scalpel injuries within the Hierarchy of Controls is below.
According to the Bloodborne Pathogens Standard, facilities must review and update their Exposure Control Plan at least annually. The facility’s reviews and Exposure Control Plan updates must also cover emerging technologies or practices to prevent sharps injuries, including safety-engineered devices.
What is required under the BBP Standard when using scalpels?
Sharps scalpels cannot be eliminated or substituted in healthcare, especially for surgical procedures. Therefore, the Bloodborne Pathogens Standard requires facilities to implement engineering controls and work practice (administrative) controls to prevent exposures to bloodborne pathogens from used scalpel blades.
Engineering Controls include the use of safety-engineered devices and sharps containers to isolate the hazard (sharp and contaminated blade) from the potentially exposed staff. Safety-engineered devices can be classified into active safety devices (e.g. safety scalpels) which need to be manually activated by the user, or passive safety devices (e.g. single-handed scalpel blade removers) which are automatically activated. Studies have suggested that passive safety devices provide superior prevention of sharps injuries to active safety devices. Some international guidelines, such as the Australian Guidelines for the Prevention and Control of Infection in Healthcare, state a preference for using passive safety devices.
Administrative Controls, which cover administering policies, work practice controls, and training requirements in facilities, help support Engineering Controls. OSHA has stated that, “As part of a comprehensive approach to managing occupational exposures to bloodborne pathogens, both work practice controls (also called administrative controls) and training are required to be part of the implementation.”
To prevent scalpel injuries under the Bloodborne Pathogens Standard, facilities must implement:
- Work-practice controls for scalpel handling, including the use of a hands-free passing technique (HFPT). Combining the use of a single-handed scalpel blade remover and HFPT has been shown to prevent half of all scalpel injuries in ORs.
- A Hepatitis B vaccination program for all potentially-exposed staff.
- Training on the risks of scalpel injuries, and the controls in place to prevent them.
What is considered an Engineering Control to prevent scalpel injuries?
The Bloodborne Pathogens Standard defines Engineering Controls as control measures that “isolate or remove the bloodborne pathogens hazard from the workplace”. Examples from the Standard include using sharps disposal containers and self-sheathing needles.
In February 2023, OSHA published a Standards Interpretation letter to clarify engineering controls in regards to scalpel blade removal. When using standard scalpels with a reusable handle and disposable blades, the use of forceps, needle holders, and re-sheathing two-handed devices to remove the scalpel blades are not considered engineering control.
According to OSHA, “a single-handed scalpel blade removal device is an engineering control for the point of disposal.”
Many facilities continue to utilise standard scalpels with a reusable handle and disposable blades as there are significant patient safety concerns with disposable “safety” scalpels. Some of the cited reasons against disposable “safety” scalpels include:
- As an active safety device, “safety scalpels” rely on the user to manually activate the safety mechanism. Research has shown that activation rates of active safety devices are inconsistent, and that passive safety devices offer superior safety.
- The design of safety scalpels impedes safe use by surgeons – the sheath can obstruct surgeon’s view, and the plastic handles can affect balance and reduce tactile feedback.
- There is a more limited choice with scalpels – the number of standard blade-and-handle scalpel combinations is significantly greater than the available safety scalpel range.
Therefore, OSHA has stated that “when the use of a scalpel with a reusable handle is required… the blade removal must be accomplished through the use of a mechanical device or a one-handed technique, such as a single-handed scalpel blade removal device.”
What do facilities need to do when choosing Engineering Controls like safety-engineered devices?
Per the Bloodborne Pathogens Standard, safety-engineered devices and other engineering controls must be chosen based on clinician feedback, and the facilities should review their chosen devices on a regular schedule (e.g. annually) to make sure that their practices are effective.
Facilities should have a representative group made up of clinical teams who handle sharps (such as surgical technicians, surgeons, perioperative nurses, etc) to evaluate available safety-engineered devices and supporting work practice controls. Where the facility is using standard scalpels with a reusable handle and disposable blades, scalpel blade removal devices should be evaluated and implemented. This group can also provide suggestions for how to effectively implement safety-engineered devices within ORs, as frontline involvement is vital to sustainable implementation.
It is also important for annual reviews of Engineering Controls to consider whether there are new safety-engineered devices available on the market, and evaluate them accordingly.
What training is required for staff exposed to sharps?
Under the Bloodborne Pathogens Standard, all staff potentially exposed to sharps must be trained, under Administration Controls. This training is required upon induction, and at least annually thereafter. OSHA requires this training to cover:
- Requirements per Bloodborne Pathogens Standard
- An explanation of the risks associated with sharps injuries, including bloodborne infection transmission
- Which tasks are higher risk of sharps injuries (e.g. surgical procedures using scalpels)
- The facility’s Exposure Control plan, and what preventative control measures have been put in place. This includes explaining the Engineering Controls, work practice controls, and PPE to be used.
- How to use any safety engineered devices being used,
- How to report sharps injuries, and the facility’s process after an injury.
- Opportunities for questions and answers.
It is important to note that if new safety-engineering devices are being implemented into facilities, training must be provided to users on how to use them.
What organisations have sharps safety guidelines to help facilities?
Professional bodies in the USA have sharps safety guidelines to assist facilities in complying the the Bloodborne Pathogens Standard. These guidelines may provide practical implementation recommendations, but facilities must ensure that they remain compliant with all requirements under the Bloodborne Pathogens Standard.
The Association of periOperative Registered Nurses (AORN) sharps safety guidelines follow the Bloodborne Pathogens standard, and provide some additional recommendations for how hospitals can implement them. AORN recommend using safety-engineered devices as engineering controls, and to use a single-handed blade remover when using standard scalpels with a reusable handle and disposable blade. They also outline the need for work practice and administrative controls including that users must not touch scalpel blades when loading and removing them. Training for how to use safety-engineered devices is also recommended per the Bloodborne Pathogens Standard, including in-person training for how to use safety-engineered devices.
The Association of Surgical Technologists (AST) sharps safety guidelines also aim to reinforce the Bloodborne Pathogens Standard. AST guidelines specify using a single-handed blade remover when using a reusable standard scalpel handle, and have some guidance for choosing safety-engineered devices. They recommend that the safety feature should be designed so that it can’t be de-activated and so that the user can easily tell that it is activated. AST guidelines refer to passive safety devices in preference to active safety devices. Like AORN, AST call for training on how to use the chosen safety-engineered devices.
Qlicksmart can help your facility be OSHA-compliant, with single-handed scalpel blade removers and sharps safety education support. To find out more, contact us today at hello@qlicksmart.com.
About the Author
Mairin Monteath is a passionate advocate for workplace safety. Since receiving their Bachelor of Communications (Mass Communications) from the University of Queensland, Mairin has worked with healthcare professionals and medical device innovators to streamline how safety solutions can be effectively implemented into healthcare facilities’ existing processes.