NHMRC’s Updated “Guidelines for the Prevention and Control of Infection in Healthcare”
The National Health and Medical Research Council (NHMRC) is Australia’s leading organisation for health and medical research. They provide funding for thousands of research projects that have benefitted people around the world. The NHMRC’s guidelines and publications are highly regarded in medical, healthcare and allied healthcare industries.
 In collaboration with the Australian Commission on Safety and Quality in Health Care, which provides the National Safety and Quality Health Service Standards (NSQHS) the NHMRC designed the all-important “Australian Guidelines for the Prevention and Control of Infection in Healthcare (2019)”. These guidelines provide a risk-management framework to ensure that the basic principles of infection prevention and control are enforced throughout Australian healthcare facilities. They are also advocated as best-practice by professional bodies including the Australian College of Perioperative Nurses (ACORN), the Australian Podiatry Association (APodA) and the Australian Dental Association (ADA).
In collaboration with the Australian Commission on Safety and Quality in Health Care, which provides the National Safety and Quality Health Service Standards (NSQHS) the NHMRC designed the all-important “Australian Guidelines for the Prevention and Control of Infection in Healthcare (2019)”. These guidelines provide a risk-management framework to ensure that the basic principles of infection prevention and control are enforced throughout Australian healthcare facilities. They are also advocated as best-practice by professional bodies including the Australian College of Perioperative Nurses (ACORN), the Australian Podiatry Association (APodA) and the Australian Dental Association (ADA).

As a safety device company, Qlicksmart has been advocating for healthcare staff safety for over 20 years, with an expertise in sharps safety. This article will focus on the April 2021 updates to the Australian Guidelines for the Prevention and Control of Infection in Healthcare, with regards to the recommendations for improving sharps safety. These update reflect the latest published data on scalpel injury prevention via the use of safety-engineered devices.
Updated: Section 3.1.2 “Use and management of sharps, safety engineered devices and medication vials”
The updated Section 3.1.2 now expands the definition of safety-engineered devices to specify the differences between active safety devices and passive safety devices.
- Passive safety devices are defined as “single handed devices where the safety device activates automatically with no extra action being required by the user.” Examples of passive safety devices include retractable spring-loaded safety syringes and a single-handed scalpel blade remover.
- Active safety devices are described as those which “require manual activation of the safety feature by the user”. Examples of active safety devices include needles with guards or safety scalpels.
- The Guidelines stipulate that “Passive safety devices should always be used in preference where appropriate to minimise risk of injury.” This is keeping with a study in 20103 that found that passive safety devices were involved in 70 times fewer injuries than active safety devices.
- The recently revised Australian Standard AS 3825:2020 Procedures and devices for the removal, containment and disposal of scalpel blades from scalpel handles, has also been added as a reference document to Section 3.1.2 of the Guidelines.
- AS 3825:2020 states that scalpel blades should not be removed by fingers, instruments not designed specifically for this task (such as artery forceps) or recapping type (two handed) devices. The Standard also stipulates that the removed scalpel blades must be immediately contained. This protects not only the user, but also downstream staff from a sharps injury with a used scalpel blade.
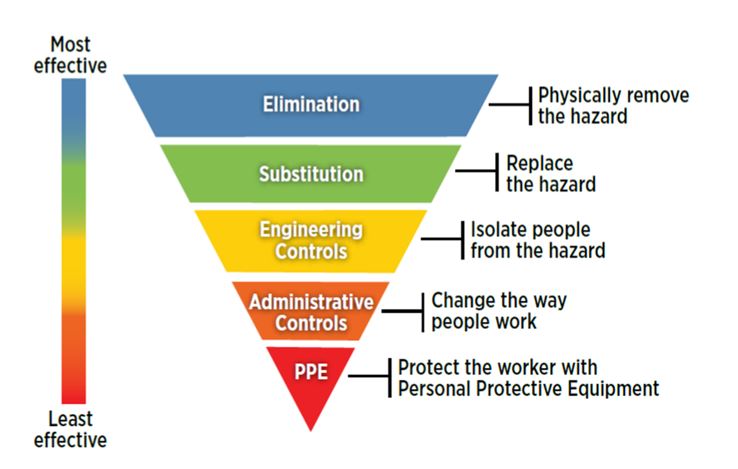
The Hierarchy of Controls is also recommended as an approach to preventing sharps injuries. The Guidelines state that “Eliminating workplace hazard and risk is a fundamental principle of all work health and safety legislation in Australia. To limit the risk of sharps injuries, the hierarchy of controls method is a well-recognised approach to prevent sharps injuries.”
 Updated: Section 8 “Glossary”
Updated: Section 8 “Glossary”
- Definitions, as found in the Glossary are:
-
- “Passive safety devices are single handed devices where the safety device activates automatically with no extra action being required by the user, such as a retractable spring–loaded safety syringe.”
- “Active safety devices, such as needles with guards, require manual activation of the safety feature by the user.”
-
NHMRC’s updated “Guidelines for the Prevention and Control of Infection in Healthcare” has a useful section that can be found in Section 11 – Amendments to the Guidelines. This section explains how and why the amendments were made. It also summarizes key details and dates of the amendments in a layout that is easier to understand.
How to Access the Latest Updates to NHMRC Guidelines:
In keeping with modern times and the rapid generation of new information, NHMRC started using MAGICapp. MAGICapp is a system that allows for continuous update of the Guidelines without the need to wait years for the next edition to be produced.
The newly updated Guidelines address the “highest level of risk of infection transmission in the healthcare setting”4 based on the latest research and evidence-based findings. Health care facilities should consider the risk of transmission of blood borne infection and implement these guidelines according to their specific setting and circumstances.
With the updates to the NHMRC’s Guidelines and the Australian Standard AS 3825 Procedures and devices for the removal and disposal of scalpel blades and scalpel handles, now more than ever, is the best time to review your facilities scalpel and sharps safety protocols.
The Qlicksmart team has resources which can help you with implementing a compliant sharps safety program in your facility. These include evaluation forms for product trials, how to use product videos, and sharps safety educational webinars.
If you have questions about the Qlicksmart’s safety devices, or our resources, contact our team by email at hello@qlicksmart.com.
References:
- Ottino MC, Argentero A, Argentero PA, Garzaro G, Zotti CM : Needlestick prevention devices: data from hospital surveillance in Piedmont, Italy-comprehensive analysis on needlestick injuries between healthcare workers after the introduction of safety devices. BMJ open 2019;9(11):e030576 Pubmed Journal
- Control CFD, P : Workbook for Designing, Implementing and Evaluating a Sharps Injury Prevention Program (2008). Centers for Disease Control and Prevention 2008; http://www.cdc.gov/sharpssafety/
- Tosini W, Ciotti C, Goyer F, et al. Needlestick Injury Rates According to Different Types of Safety‐Engineered Devices: Results of a French Multicenter Study. Infection control and Hospital epidemiology 2010;31(4):402-07
- Australian Guidelines for the Prevention and Control of Infection in Healthcare (2019). NHMRC 2019; http://www.nhmrc.gov.au/about-us/publications/australian-guidelines-prevention-and-control-infection-healthcare-2019


