Sharps Injuries in Laboratories
Sharps injuries are a significant injury and health hazard for those that work in laboratories, especially in “wet labs” where they are exposed to hazards such as blood and chemicals. These hazards have the potential to transmit blood borne infection (BBI) that can have a serious impact on health. In addition, the anxiety and side effects of post-exposure have a significant impact on laboratory workers, with an infection having the potential to limit their future career prospects.
Scalpel injuries in particular are estimated to cost between USD 500 – USD 3000 for an uncomplicated injury (1), and over USD 15,000 for a complicated injury that requires surgery. If exposed to BBI, treatment costs are a lifetime continuing cost.

Sharps Injury Statistics
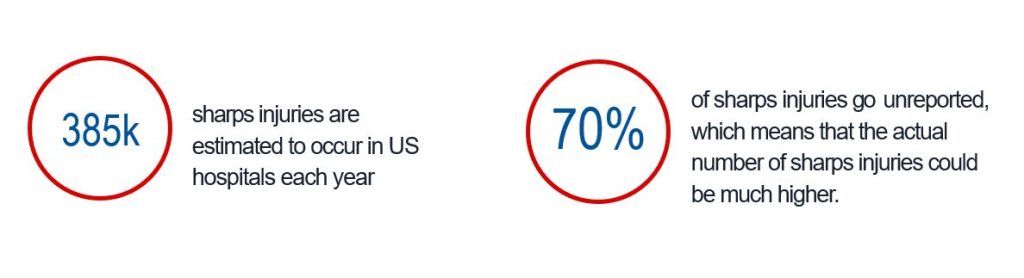
It is estimated that in US Hospitals, 385,000 sharps injuries occur each year (2) with 70% of those injured go unreported (3).
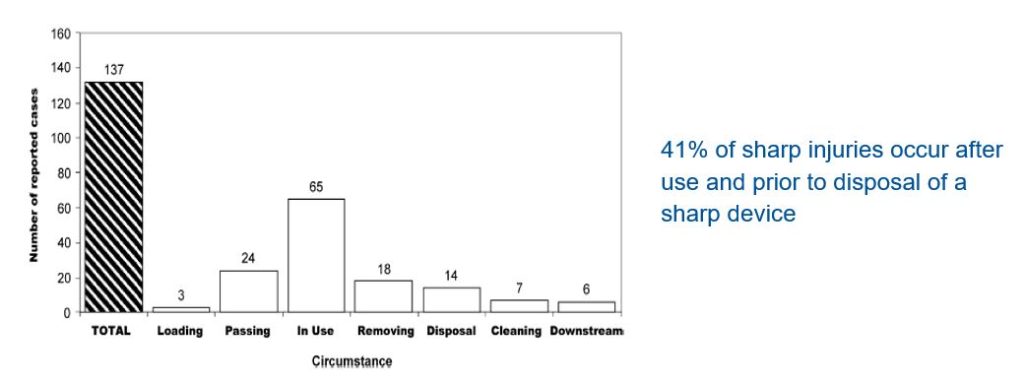
Clinical data suggests that 41% of sharps injuries occur after use and prior to disposal of a sharps device (4).
Occupational Safety and Health Administration
The Occupational Safety and Health Administration (OSHA) has created a Bloodborne Pathogen Guideline that help prevent the occurrences of sharps injuries. In particular, they have recommended that where engineering controls are commercially available and feasible, they must be used (5).
OSHA has further stated that:
“… using fingers to remove a used scalpel blade does not meet the requirements of the standard.”
“Some facilities use a twohanded procedure with hemostat as a mechanical device to remove scalpel blades… Hemostats have been used as a measure which was preferable to using fingers to remove a used scalpel blade. Employers are expected to consider and use safer and more effective measures when feasible.”
“… suggestion that the BBP (bloodborne pathogen) standard be changed to require that if a mechanical device is utilized, it must be a ‘onehanded use of a mechanicaldevice’ is a very good recommendation and one that would improve worker safety.”
“The use of a singlehanded scalpel blade remover meets these criteria”
Qlicksmart’s BladeFLASK Scalpel Blade Remover
As a single-handed blade remover, Qlicksmart’s BladeFLASK and BladeFLASK EVO Scalpel Blade Remover are the best option for use in laboratories.
-
- BladeFLASK can be mounted on stations
- BladeFLASK works within Standard Operating Procedures
- Fast implementation process
- Less training (compared to using other devices)
- Compatible with wide range of blades
- BladeFLASK improves safety
- In university space (6)
- BladeFLASK can fit under budget for engineering controls, PPE, or consumable

The BladeFLASK Scalpel Blade Remover is now being used at university laboratories, pathology, medical, and research labs around the world to reduce sharps injuries. The new BladeFLASK EVO is even better as it can accommodate more types of scalpel blades and handles giving the user better freedom in choosing the scalpel that they want.
Case Study
In a study at the University of St Andrews Scotland UK over a 5-year period, for every 1000 hours of scalpel usage in a dissecting laboratory there were 35 injuries (6). The authors conclude that, “The following factors have contributed to increased sharps safety influencing frequency rates: single-handed blade removal systems; mandatory personal protective equipment; and having only one student dissecting at a given time.”
Qlicksmart’s single handed blade removers can be seamlessly integrated into a laboratory’s safety curriculum:
- Use of a scalpel blade removal system mounted in several stationary points within the laboratory increasing its accessibility scalpel blade removal can be completed at point of use
- The count is made easy with clear cartridges
- Automatic containment of used scalpel blades ensures that all students are protected from injury
To find out more about organising a trial for your laboratory with Qlicksmart safety devices, send us an email at hello@qlicksmart.com.
References
- Kanamori H, Weber DJ, DiBiase LM, Pitman KL, Consoli SA, Hill J, Sickbert-Bennett EE, Rutala WA: Impact of Safety-Engineered Devices on the Incidence of Occupational Blood and Body Fluid Exposures Among Healthcare Personnel in an Academic Facility, 2000–2014. Infection Control & Hospital Epidemiology 2016, 37(5):497-504
- Centers for Disease Control and Prevention. Workbook for Designing, Implementing, and Evaluating a Sharp Injury Prevention Program. Available at: http://www.cdc.gov/sharpssafety/pdf/sharpsworkbook_2008.pdf
- Osborn, E.H.S., Papadakis, M.A., & Gerberding, J.L. (1999). Occupational exposures to body fluids among medical students: a seven-year longitudinal study. Annals of Internal Medicine, 130, 45-51.
- Fuentes, H., et al. (2008). “”Scalpel Safety”: Modeling the effectiveness of different safety devices’ ability to reduce scalpel blade injuries.” The International Journal of Risk & Safety in Medicine20(1-2): 83-89.)
- Occupational Safety and Health Standards 1910.1030 – Bloodborne Pathogens. Occupational Safety and Health Administration. US Department of Labor. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030
- Foytl J, Chisholm F, Varsou O. Sharps Injuries during Dissection: A Five-Year Retrospective Study in the Context of Safety. Anat Sci Educ. 2020 Mar;13(2):158-167. doi: 10.1002/ase.1894. Epub 2019 Jun 10. PMID: 31091009

